A Tale of Two MARDs
Apples and oranges?
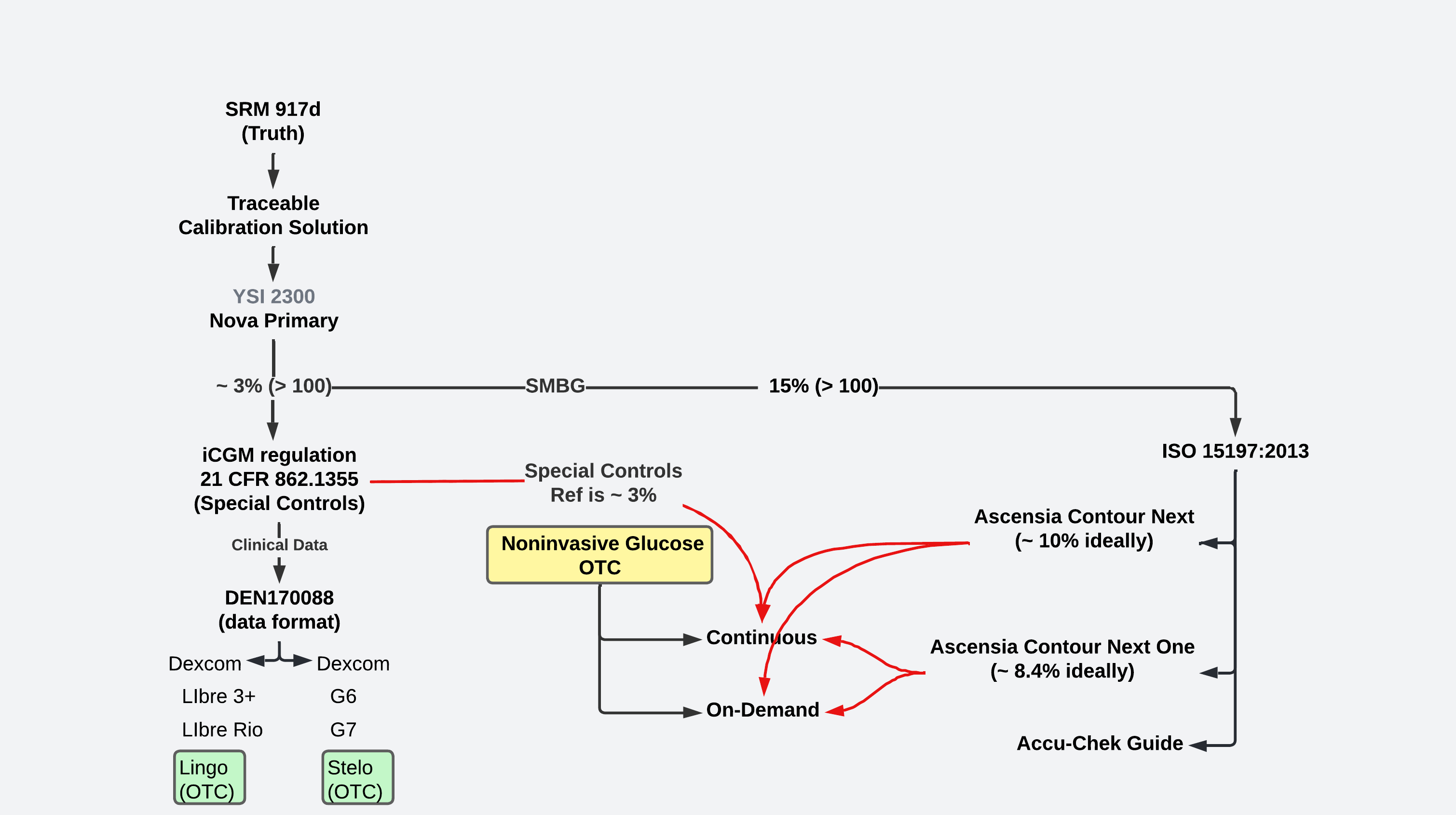
The technology at the core of the over-the-counter continuous glucose monitors from Dexcom and Abbott were cleared by FDA as meeting the integrated continuous glucose monitor (iCGM) regulation: 21 CFR 862.1355. Both iCGMs are factory-calibrated. Both are available without prescription for about $3 per day. Both sensors were clinically studied with a YSI 2300 as a reference (monitor). Published MARDs for the core technologies were computed using YSI 2300-paired samples.
When operated by a skilled technician, the YSI 2300 is capable of presenting glucose measurements that are within about 3% of the standard material: SRM 917d. The YSI 2300 has been retired by its manufacturer. Nova Biomedical manufactures a substantially-equivalent Nova Primary to fill the void created by the absence of the YSI 2300. The Pros: The FDA accepts it as a replacement. The cons: It's costly. The sample size is approximately 25 uL (compare to a 0.6 uL sample for Ascencia's Contour Next), and it requires skilled technicians for operations. For reference, a drop of blood is approximately 50 uL. Samples drawn for the Nova Primary are more painful than a samples drawn for an Ascensia Contour Next.
Noninvasive Reference Options
The choice of a reference (monitor) for the over-the-counter noninvasive glucose methods presents options:
- A Nova Primary (~ 3%); or
- An Ascensia Contour Next (~ 10%, when used by a skilled person); or
- An Ascensia Contour Next One (~ 8.4%, when used by a skilled person)
As noninvasive researchers obtain preliminary paired data, there may be an urgency to report progress as MARD and draw comparisons to the MARDs reported by Abbott and Dexcom. Most startups lack access to a Nova Primary. Ascensia's Contour(R) Next One is popular as a reference method for noninvasive adventures.
Abbott and Dexcom have set the bar for cgm MARD from pivotal clinical studies at less than 10%. We can expect noninvasive stories to target a MARD of less than 10%, perhaps presenting a MARD directly comparable to the iCGMs from Abbott and Dexcom.
A MARD calculation using other than a lab reference is not comparable to the MARDs reported for clinical data by the current iCGMs on the market.
Noninvasive methods operating on-demand, initiated by the user to obtain a value, are more similar to fingerstick methods measured against ISO 15197:2013, e.g., Ascensia's Contour(R) Next One. On-demand noninvasive glucose predictions are not continuous glucose monitoring.
